Polyethylene Manufacturing Process
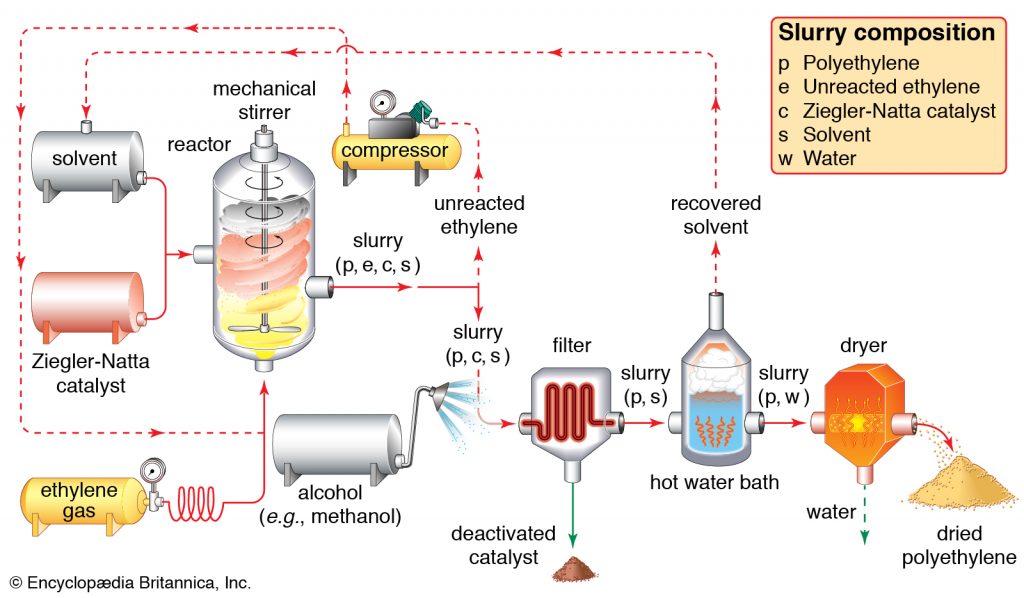
Polyethylene (PE) is a light, flexible synthetic resin derived from ethylene polymerization. It is the world’s most frequently used plastic, found in everything from clear food wrap and shopping bags to detergent bottles and automotive fuel tanks. It can also be sliced or spun into synthetic fibers or made to have rubber-like elasticity.
Chemical Composition & Molecular Structure
Ethylene (C2H4) is a gaseous hydrocarbon frequently created by splitting ethane, a major component of natural gas and can also be distilled from petroleum. Ethylene molecules comprise two methylene units (CH2) joined together by a double bond between the carbon atoms, resulting in CH2=CH2. The double bond can be broken under the influence of polymerization catalysts. The resulting additional single bond can be used to bind to a carbon atom in another ethylene molecule. Ethylene has the following chemical structure when it is formed into the repeating unit of a big, polymeric (multiple-unit) molecule:
-CH2-CH2–
The properties of polyethylene are determined by this basic structure, which is repeated thousands of times in a single molecule. Long, chain-like molecules with hydrogen atoms linked to a carbon backbone can be made in either linear or branching forms. Low-density polyethylene (LDPE) or linear low-density polyethylene (LLDPE) are branched varieties, whereas high-density polyethene (HDPE) and ultrahigh-molecular-weight polyethene are linear versions (UHMWPE).
Low-Density Polyethylene

In the presence of oxide initiators, LDPE is made from gaseous ethylene at very high pressures (up to about 350mp, or 50,000 pounds per square inch) and high temperatures (up to about 350 °C [660 °F]). These procedures produce a polymer with both long and short branches.
LDPE is a very flexible polymer because the branches prevent the polyethene molecules from clustering tightly in rigid, stiff, crystalline formations. It has a melting point of around 110 °C (230 °F).
Packaging film, garbage and grocery bags, agricultural mulch, wire, and cable insulation, squeeze bottles, toys, and housewares are some of the most common applications.
Linear-low Density Polyethylene

LLDPE has a similar structure to LDPE. It’s manufactured by employing Ziegler-Natta or metallocene catalysts to copolymerize ethylene with 1-butene and minor amounts of 1-hexene and 1-octene. The resulting structure has a linear backbone but short, uniform branches that, like LDPE’s longer branches, hinder the polymer chains from packing tightly together. LLDPE shares many qualities as LDPE and competes in the same markets. The key advantages of LLDPE are that the polymerization conditions are less energy-intensive and that the properties of the polymer may be changed by changing the kind and amount of chemical components.
High-Density Polyethylene

HDPE is made utilizing Ziegler-Natta and metallocene catalysts and activated chromium oxide at low temperatures and pressures (known as a Phillips catalyst). Because it lacks branching, the polymer chains can pack tightly together, resulting in a dense, highly crystalline material with high strength and mild stiffness. With a melting point of more than 20 degrees Celsius (36 degrees Fahrenheit) higher than LDPE, it can tolerate repeated sterilization at 120 degrees Celsius (250 degrees Fahrenheit). Blow-moulded milk and household cleaner bottles, as well as blow-extruded supermarket bags, construction film, and agricultural mulch, and injection-moulded pails, caps, appliance housings, and toys, are among the company’s offerings.
Ultrahigh-molecular-weight Polyethylene
Ultrahigh-molecular-weight forms of linear polyethene can be made, with molecular weights ranging from 3,000,000 to 6,000,000, compared to 500,000 atomic units for HDPE. These polymers may be spun into fibres and then pulled or stretched into a highly crystalline form, giving them exceptional stiffness and tensile strength that rivals steel. Bulletproof vests are created using yarn made from these fibres.
Pros
- Polyethene uses far less water than alternatives such as paper and cloth.
- It is less expensive than other options.
- The strength and durability are great.
Cons
- If we keep using them at our current rate, by 2050, there will be approximately 1000 million tons of plastic in the ocean, and it will take thousands of years to undo the damage we have caused.
